The NHS recently announced the nationwide roll-out of an innovative diabetes treatment, designed to automate insulin management and sustain healthy blood glucose levels. We detail how this can transform the lives of people who suffer from type 1 diabetes.
REVOLUTIONARY ROLL-OUT OF INNOVATIVE DIABETES TREATMENT
A landmark announcement by the National Institute of Health Care and Excellence (NICE) has outlined details of who will be offered hybrid closed-loop (HCL) technology to treat type 1 diabetes in England and Wales, and how it will become established over the coming years.
Thousands of people living with the condition could be offered the wearable technology to assist them in managing their health, following the publication of the guidance from NICE earlier this month.
Often recognised as an ‘artificial pancreas’, the HCL systems work by linking an insulin pump and a continuous glucose monitor (CGM) with a computer algorithm.
By continuously monitoring glucose levels in the body, the innovative technology can automatically administer the correct amount of insulin, based on blood sugar readings. In doing so, the HCL system is constantly able to keep blood glucose levels within a safe and healthy range.
It is hoped that a mass roll-out of the HCL system for type 1 diabetes sufferers in the UK will decrease the need for regular finger prick testing, or the necessity for self-administered insulin injections to control blood sugar levels, due to the automated nature of the technology.
Manual input will still be required in some cases, such as when eating or doing exercise. However, compared to widely used diabetes management treatments, such as insulin injections and carbohydrate counting, the freedom and autonomy that the HCL system offers is second to none.
In short, this new technology will be life-changing for many long-term sufferers of type 1 diabetes.

ADDRESSING A HOST OF ISSUES
Those who suffer from type 1 diabetes are constantly at risk of becoming hyperglycaemic or hypoglycaemic, depending on their blood sugar levels. This occurs when a person’s blood glucose levels become too high (hyper) or too low (hypo).
Blood glucose levels can only be regulated by administering insulin. The aim of treatment is to control hyperglycaemia due to there being little or no insulin production from the pancreas, and avoid hypoglycaemia, as both extremes are considered dangerous.
Left untreated or poorly controlled, type 1 diabetes can lead to long-term health complications including disabling hypoglycaemia, strokes, heart attacks, blindness, amputations, and kidney problems.
Around 10 percent of the entire NHS budget is currently spent on treating diabetes and associated health complications. At a time of national economic strain, this figure presents an important issue to address.
Therefore, it is paramount that NICE focuses on obtaining and developing the most cost-effective and efficient technologies available.
By investing in HCL systems, NICE seeks to present type 1 diabetes sufferers with a long-term, affordable treatment option, capable of keeping their blood glucose levels within the recommended range without constant intervention.
As a result, people with type 1 diabetes are less likely to develop complications, saving the NHS money in the long term.
Meanwhile, the question of equality and fair distribution has been debated since the draft recommendations for the roll-out were introduced by NICE in January this year.
Initially, the HCL system was to be offered to those that struggle to maintain HbA1c levels of 6.5 percent (48 millimoles per mole (mmol/mol) or below). In January, Diabetes UK called for this figure to be adjusted to include those who have an HbA1c of 7.5 percent (58 mmol/mol) or higher – taking into consideration risks associated with hypoglycaemia and broadening the eligibility criteria for patients overall.
Furthermore, Diabetes UK and other charities have called for people with disabilities and their carers to be educated on how to use the HCL system safely. An approved structured education programme was subsequently proposed to implement this knowledge.
The most recent NICE recommendations gladly reflect these changes, as will the upcoming final publication of the guidance in December 2023.
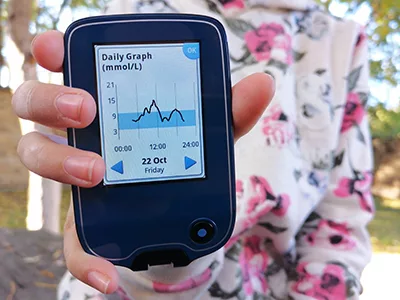
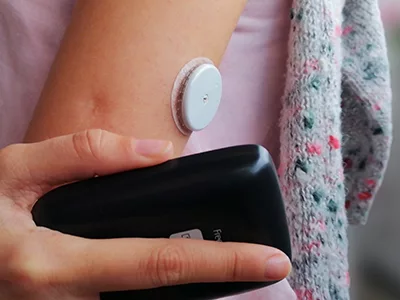
HCL FUNDING
Currently, a single HCL insulin pump can cost between £2,000 and £3,000. Additionally, essential equipment required for its yearly upkeep costs around £1,500; individuals who suffer from type 1 diabetes could therefore be spending up to £4,500 per year on the technology.
However, despite the considerable costs of HCL technology, NICE assessed that the treatment still results in considerable cost savings over the course of a patient’s lifetime, due to a reduced risk of commonly associated conditions such as blindness or kidney failure. It is also proven to extend life expectancy.
Following NICE’s proposed public roll-out of HCL technology in England and Wales, the NHS has announced funding worth £2 million for a pilot run. This funding equates to approximately 1,000 eligible people living with type 1 diabetes in England being offered the HCL technology on the NHS.
Yet, there are over 150,000 living with type 1 diabetes in England alone. Many believe that significantly more funding must be made available to supplement the offering of HCL on the NHS.
With the help of over 2,000 campaigners, Diabetes UK, alongside others, called for additional funding to be provided to support the roll-out.
Recent funding campaigns seek to enable the NHS to offer HCL systems widely, fairly, and sustainably. Those fighting this issue wish to avoid potential geographical bias seen in roll-outs of the past, such as the implementation of the insulin pump in the 1970s, and overcome possible limitations to treatment access.

WHAT HAPPENS NEXT?
NICE’s recommendations are the result of a three-year process that involved extensive consultation with stakeholders and medical trials to assess the benefits of the technology, but these recommendations are just the beginning.
The HCL system means significant changes to the way specialist diabetes services are delivered. To manage workforce pressures and ensure people are supported in using the technology safely, a phased five-year implementation plan has been developed.
Managing the roll-out gradually is designed to help the NHS deliver the technology in a way that offers the greatest benefit to people who are most in need of the treatment.
As part of this plan, priority will initially be given to children and young people under the age of 18 and women who are pregnant or planning a pregnancy – where the need to optimise care is often highest. Existing insulin pump users interested in upgrading to HCL technology will be accommodated once there is a greater capacity to treat more people.
This means that not everyone eligible will have immediate access to the HCL system, however, the phased implementation of the technology is set to be managed in a way that will ensure fairness and adapt to local circumstances.
The roll-out will also be subject to regular monitoring through an oversight group set up by NHS England. Diabetes UK will be part of this group, which has been established to assess data and create reports to ensure that access and uptake of HCL is fair and not driving inequalities.